Dr Paul Lumu, MAAIF Uganda. Picture (c) P. Bastiaensen (woah) 2025.
The fifth session of the Standing Group of Experts on African Swine Fever (ASF), held in Lomé, Togo, focused on vaccines and vaccination strategies. Participants included representatives from the World Organisation for Animal Health (WOAH), the Food and Agriculture Organization (FAO), and the African Union agencies, among others.
Dr. Abebe Wolde (FAO, Accra) presented a general overview of the ASF situation in Africa, highlighting its impact on livelihoods and food security, particularly affecting smallholder farmers. He emphasized the challenges of controlling ASF without a vaccine, including weak surveillance systems, poor biosecurity practices, and informal pig trade.
Dr. Wolde also discussed FAO’s support in promoting regional cooperation, early detection, and biosecurity measures to combat the disease. The presentation underscored the need for improved diagnostic capacities and a shift towards more secure pig production systems to effectively manage ASF in Africa.
Dr. Viola Chemis (WOAH, Nairobi) presented the outcomes of the Global Coordination Committee meetings, highlighting the need for country-specific strategies, capacity building, and improved surveillance in Africa.
Dr. Pam Luka from the GARA Africa Chapter (GAC) detailed GARA-Africa’s research efforts and collaboration across 16 African countries to address ASF, emphasising the need for genotype-specific vaccines, appropriate for Africa, and improved surveillance.
The meeting was attended by representatives of the following SGE Members :
Overall 58 participants attended the meeting, with 50% online participation. Thirty percent of participants (online and in-person combined) were women.
FLTR : Drs Sabenzia Wekesa (VS, Kenya), Mohammed Shamsuddin (FAO) and Karim Tounkara (WOAH). Picture (c) P. Bastiaensen (woah) 2025.
Dr Charles Bodjo, ag. Director of the African Union Pan-African Veterinary vaccines Centre (AU-PANVAC) discussed the importance of evaluating ASF vaccines on the continent and the challenges posed by the virus’s genetic diversity.
Prof. Emmanuel Couassy-Hymann, President of the WOAH Biological Standards Commission (BSC) presented the latest WOAH standards for ASF vaccine development, outlining the minimum requirements for safety and efficacy.
Dr Gregorio Torres, Head of the WOAH Science Department thereafter presented the guidelines for field evaluation and post-vaccination monitoring, emphasizing the importance of surveillance and data sharing for vaccine effectiveness.
Dr María del Carmen (« Carmina ») Gallardo Frontaura of the WOAH Reference Laboratory for ASF at the Spanish Centro de Investigación en Sanidad Animal (CISA), Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), provided an update on the global vaccine situation, highlighting the need for countries to define their needs and objectives before implementing vaccination programmes. Indeed, ASF has now spread to 68 countries and affected over 1 million pigs since 2022. Dr. Gallardo explained that early detection has more than once failed and the disease often stays ahead of control measures, leading to the need for effective prevention strategies including vaccines.
Dr Carmina detailed the history of ASF vaccine development, noting that while live-attenuated vaccines showed promise with 90% protection in the 1960s, they were abandoned due to severe side effects and recombination events.
Cover : FAO white paper on Considerations for competent authorities and agricultural industries on use of African swine fever vaccines (Genotype II) to enhance disease control.
More recent efforts in Vietnam and other countries have resulted in commercial vaccines based on genotype II strains, achieving 93-95% protection in the field, though these vaccines face challenges including limited cross-protection against different genotypes (of relevance to Africa), lack of DIVA-compatible tests, and risks of reversion to virulence.
Dr. Andriy Rozstalnyy, Animal Health Officer of the UN Food and Agriculture Organisation (FAO, Rome) presented the white paper on the use of ASF vaccine genotype tools for disease control, highlighting challenges in vaccine development and the importance of genomic surveillance.
Dr Douglas Gladue, formerly with USDA-ARS and now with SEEK Labs shared findings on vaccine development and cross-protection studies, emphasising the need for regionally specific vaccines.
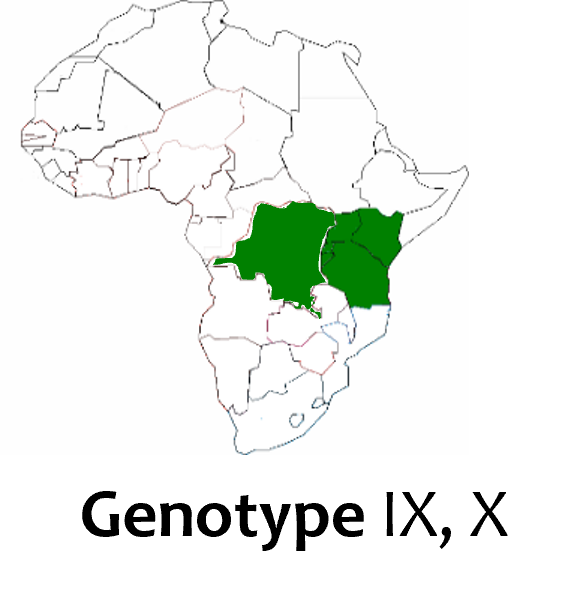
Finally Drs Anna Lacasta-Marin and Hussein Abkallo from ILRI presented promising results on a new ASF vaccine candidate generated using gene editing technology, offering 100% protection against genotype IX, one of the most commonly encountered genotypes in eastern Africa (see map on the right).
The presentations sparked discussions on vaccine development, safety, and the need for surveillance and tailored approaches to control ASF in Africa.
Based on a guided discussion moderated by Dr Juanita Van Emmenes from the WOAH Reference Laboratory for ASF at the Onderstepoort Veterinary Research institute in South Africa, participants discussed the development and implementation of vaccines for African Swine Fever (ASF) for Africa.
The group decided to continue encouraging research through partnerships with organisations like AU-PANVAC and ILRI, while continuing to emphasise the importance of biosecurity and movement control measures in the meantime. They also agreed to develop a clear communication strategy to inform countries about vaccine use and risks.
On behalf of H.E Dr. Moses Vilakati, the Commissioner for the Department of Agriculture, Rural Development, Blue Economy, and Sustainable Environment (DARBE) of the African Union Commission, I bring warm greetings to the Government and the People of the Republic of Togo, GF-TADs Africa Members and to welcome you all to the 5th Fifth Meeting of the Standing Group of Experts (SGE) on African swine fever (ASF) of the GF-TADs for Africa followed by the Regional Workshop on Coordination Mechanism, Partnership, and Advocacy for Resource Mobilization to Implement the Regional ASF Control Strategy.
First of all, I would like to congratulate E. Antoine Lekpa Gbegbeni for his appointment as the new Minister for Agriculture, Fisheries, Animal Resources and Food Sovereignty of the Republic of Togo; Congratulations and best wishes for our continued collaboration and partnership.
I wish also to take this opportunity to express AU-IBAR’s appreciation to the Food and Agriculture Organization of the United Sates (FAO), the World Organisation for Animal Health (WOAH), the International Livestock Research Institute (ILRI) for the exemplary and longstanding partnership in organising this 5th ASF SGE meeting and the Regional Workshop on Coordination Mechanism, Partnership, and Advocacy for Resource Mobilization to Implement the Regional ASF Control Strategy workshop and for standing with us in many regional and continental initiatives in Animal Resources development in Africa. Please allow me to also appreciate the commitment and the interest of the Government of the Republic of Togo in the animal and fishery resources sector which is one of the pillars of its economy. I thank you all for the individual and collective efforts towards making this important event a success.
Honorable Minister, Distinguished Participants
Pig production is increasing in most parts of Africa, contributing to better livelihoods for millions of families and supporting several of the sustainable development goals. The potential of the sector is however hampered by under-financing compared to other agricultural sectors as well as occurrence of diseases, including African swine fever (ASF).
However, ASF continues to challenge our efforts to ensure food security, economic stability, and rural development. Its devastating impact on pig populations, farmers, and communities across the continent cannot be overstated. This underscores the urgency for coordinated, science-based actions that this workshop is gathered here to address for the last 3 days. Many successful interventions to address animal health challenges have been undertaken in the past by Member States, inter-governmental institutions including AU-BAR, RECs, WOAH, FAO, Development partners and Research Institutions such as ILRI to mention just a few. These collaborative and concerted interventions have had positive impacts in addressing ASF and many animal health related issues and they have enhanced the livelihoods of animal resource dependent communities and national economies.
Dear Colleagues
The TADs challenges including ASF that confront African region are numerous and no one institution or country can address them alone. Smart Partnerships afford us an opportunity to tackle issues of mutual concern in a concerted and unified manner. This is the only way to maximize our resources for amplified impact. It is in this regard that I commend the partnership between the AU Specialized Technical Offices (STO) namely AU-IBAR and AU-PANVAC ; and FAO, WOAH, AU-PANVAC and ILRI among others.
Mobilizing resources for effective implementation of ASF control strategy in Africa requires a multi-faceted approach. This includes
(i) Building Partnership through fostering collaboration with international organizations FAO, WOAH and other stakeholders to leverage resources, expertise and funding;
(ii) Secure funding: Identify and mobilize financial resources from governments, donors and private sector organizations to support ASF control Efforts;
(iii) Capacity building: invest in training programs for veterinary services, farmers, and other stakeholders to enhance their ability to prevent, detect and respond to ASF outbreaks;
(iv) Support research and development to encourage research in ASF vaccines, diagnostics and other tools to enhance control efforts; and
(v) Coordinate efforts to ensure coordination among countries, RECs and partners to avoid duplication of efforts and maximize impact
Distinguished ladies and gentlemen,
I commend the theme of this 5th ASF SGE “ASF vaccines and vaccination”. This reflects the disease situation on the landscape and our collective aspirations in line with the Regional Strategy for the Control of ASF in Africa. As for now, No cure or effective and safe vaccines exist. Therefore development of vaccine for mass vaccination of igs is highly expected and will definitively curb the disease spread and safeguard the growing pig industry in Africa. I assure you that AU-IBAR together with AU-PANVAC remains committed to supporting all countries and RECs in the fight against priority animal diseases such ASF and others and will continued to maintain strategic partnerships with FAO, WOAH, the RECs and all stakeholders inclusive to deliver the needed support to our Member States.
I look forward to continued partnerships with all stakeholders in our collective pursuit to improve animal health and welfare in all Member States. By working together and leveraging resources, AU-IBAR, Countries, RECs and Partners can effectively control ASF and mitigate its impacts on African Pig Industry and food security.
And with these remarks, I wish you all very pleasant and successful deliberations. I thank you very much for your kind attention
PDF - 873.39KB
PDF - 670.60KB
PDF - 1.21MB
PDF - 2.48MB
PDF - 590.66KB
PDF - 10.15MB
PDF - 666.43KB
PDF - 905.99KB
PDF - 3.60MB
Delegations from Cameroon (left) and Democratic Republic of Congo (centre and right). Picture (c) P. Bastiaensen (woah) 2025.
The meeting ended by agreeing on the following draft action points (to be revised and finalised within two weeks, following the meeting) :
As and when ASF vaccines are made available:
Regarding future SGE membership, participants discussed potential new Members (countries) and (support) laboratories, noting that some current members had not been participating in meetings lately. The group agreed to give non-participating members one final chance to engage before considering substituting them:
They also briefly touched on the timing (2026) and format (likely online, unless funding becomes available) of future meetings. The topic of the next meeting will be socio-economics, i.e. regional ASF risk assessment and socio-economic impact for effective prevention, control and evidence-based advocacy. As requested by the members, compensation will be included in the agenda.