Responsible and prudent use of antimicrobial agents is part of good veterinary and animal husbandry practices. OIE has spearheaded the initiative to build a Global Database on Antimicrobial Agents Intended for Use in Animals, in alignment with the Global Action Plan on Antimicrobial Resistance.
The Fourth Annual Report on antimicrobial agents intended for use in animals provides an analysis for the global understanding of antimicrobial agent use in the animal sector. It highlights the increased capacity for country surveillance and accurate collection of data and establishes baselines for countries to monitor the implementation of national regulatory framework. The contribution of the African countries has been integral to the attainment of the global result.
The sub-optimal use of therapeutic antimicrobials for animals, a practice widely common in Africa, enhances the development of AMR. Efforts to ensure that the medicines are administered to the animals only when needed and at the correct therapeutic levels could be strengthened by the enforcement of the legislation on regulation of veterinary medicine.
In animal health, there is need to establish cooperation between the stakeholders in the animal health industry, veterinarians, veterinary paraprofessionals and farmers to promote prudent use of antimicrobials.
Dr. Dooshima Kwange, OIE AMR and Veterinary Products focal point, Federal Ministry of Rural Development, Nigeria.
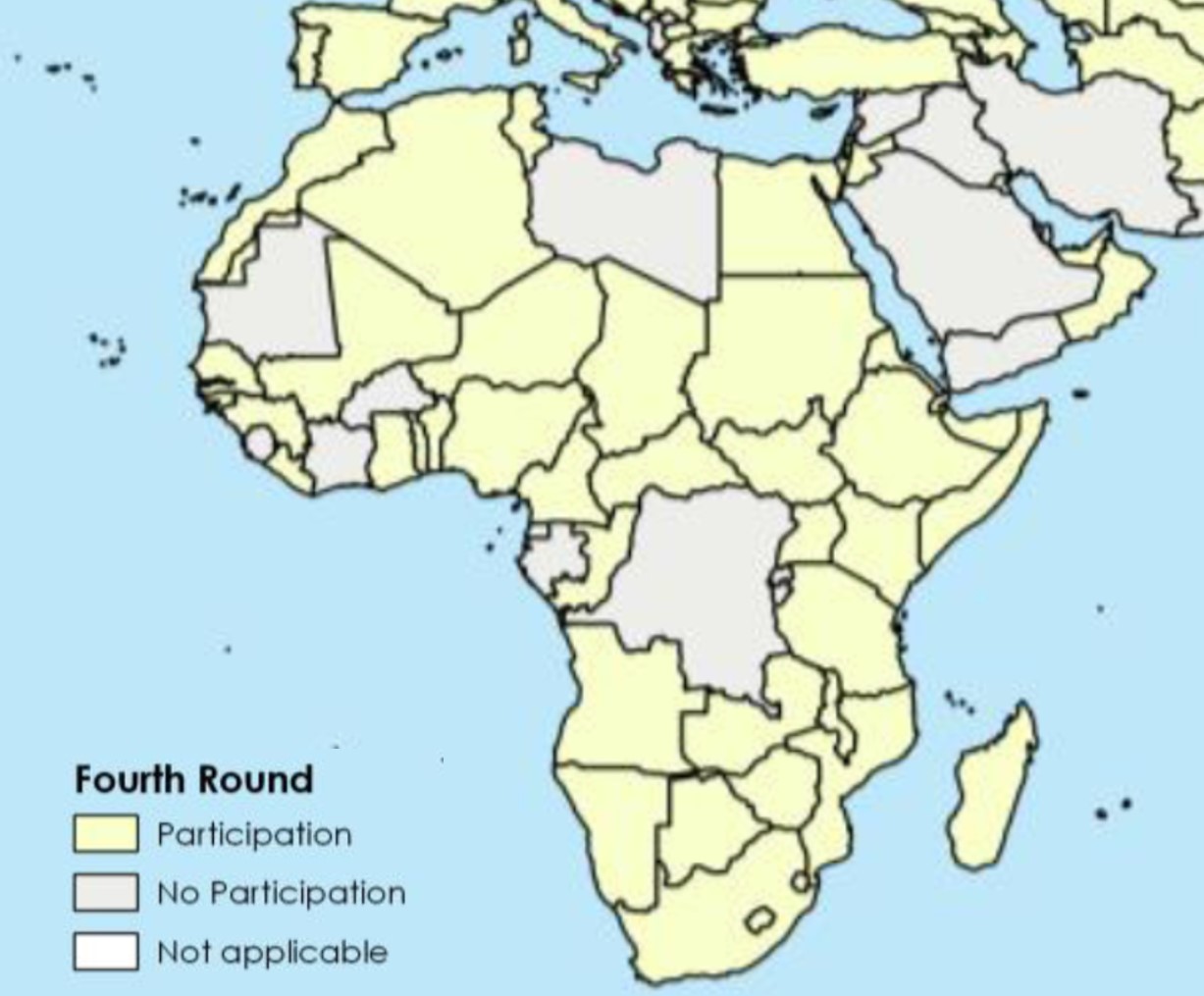
Distribution of OIE Members in Africa that responded to the OIE survey in the fourth round of data collection
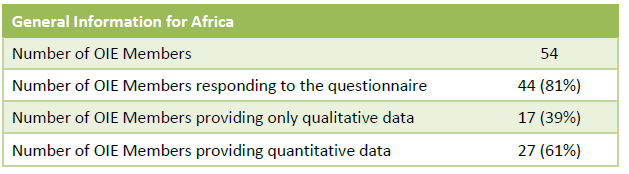
General Information for Africa During the Fourth Round of Data Collection
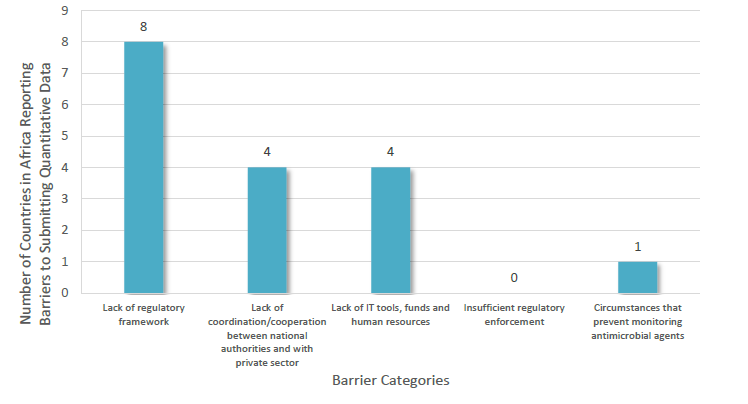
Country Barriers to Reporting Quantitative Data on Antimicrobial Agents Intended for Use in Animals in 13 Countries in Africa During the Fourth Round of Data Collection
After the fourth round of data collection, to facilitate addressing the barriers to providing quantities of antimicrobial agents in animals, a regional workshop was conducted on the OIE data collection database in Eastern and Southern Africa, in Mombasa, Kenya on the 29th to the 31st of October 2019.
By bringing together OIE Focal Points for Veterinary Products, the AMR focal point from the animal sector (if different from the OIE Focal Point for Veterinary Products) and a representative of the national drug regulatory authority the workshop sought to enhance collaboration and support inclusion of AMU as an important component of countries NAP on AMR.
In the future, the OIE will develop a software solution for the annual data collection which is expected to integrate calculations and error detection mechanism to ensure better data quality and readily accessible data and be a dynamic data analysis tool.
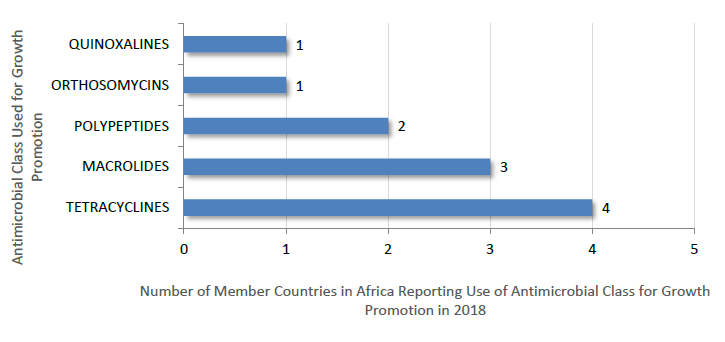
Antimicrobial Growth Promoters Used in Animals in Countries in Africa in 2016
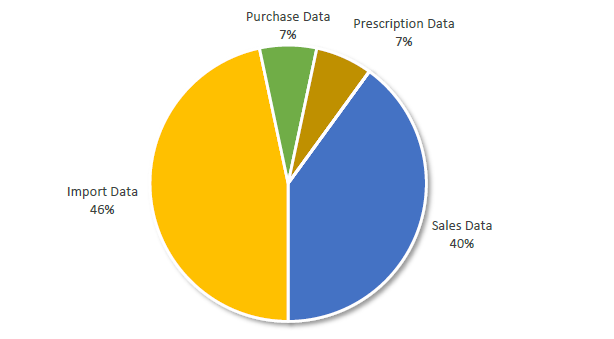
Data Sources Selected by 16 African OIE Members Reporting Quantitative Information for 2016
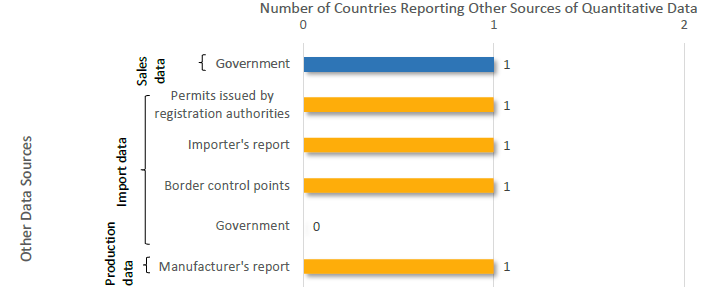
‘Other’ Source of Data as Explained by 4 Members in Africa Reporting Quantitative Information for 2016
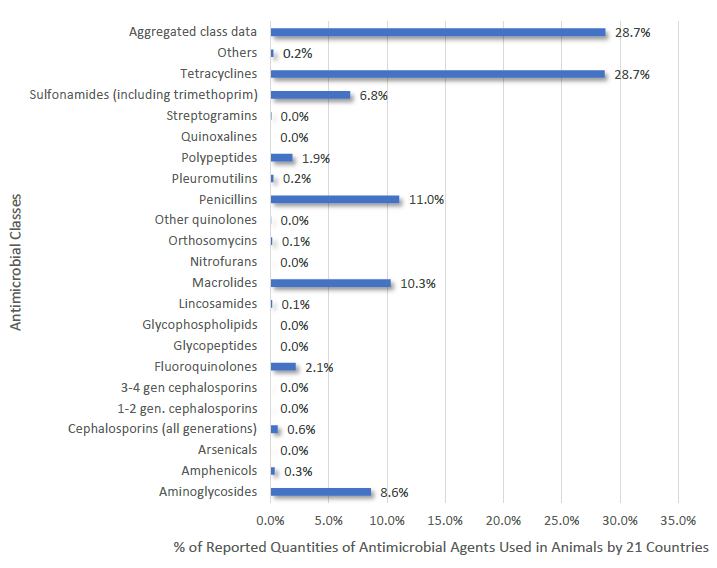
Proportion of Antimicrobial Classes Reported for Use in Animals by 21 African Members in 2016
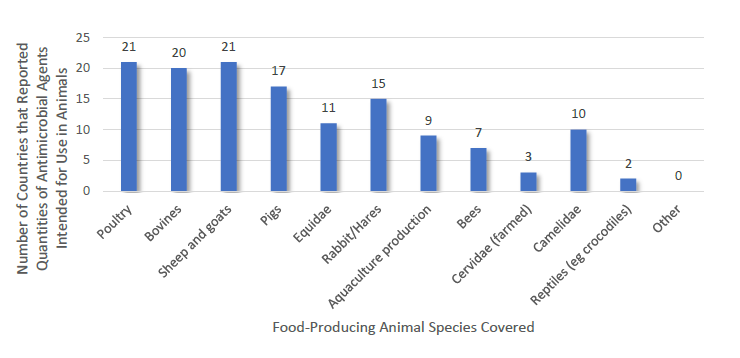
Food-Producing Animal Species Included in Quantitative Data Reported by 21 African Members in 2016
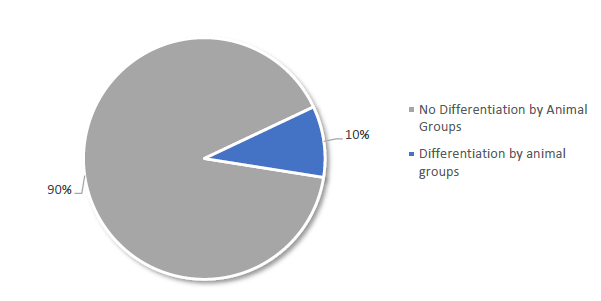
Differentiation by Animal Groups Among 21 Members in Africa Reporting Quantitative Data in 2016
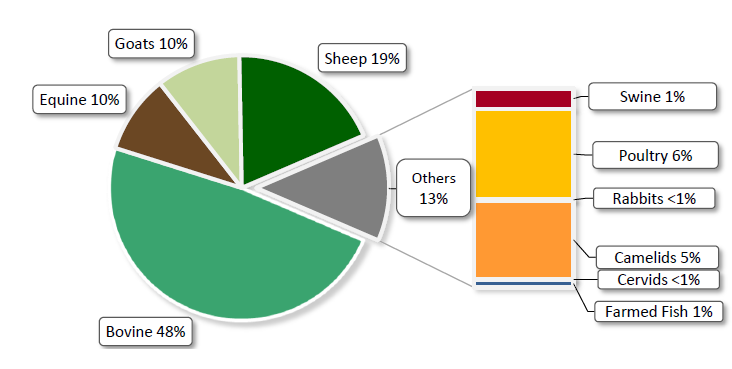
Species Composition of Animal Biomass for the 21 Countries in Africa Included in 2016 Quantitative Data Analysis
In Africa, the mg/kg estimate for 2016 for 21 countries is 39.17 mg/kg, with an upper level estimate of 45.25 mg/kg when adjusted by estimated coverage.
The OIE aims to continue working collaboratively with African country governments to strengthen their capacity to monitor and regulate the use of antimicrobials, improve awareness of antimicrobial resistance and support them to adopt the OIE Standards to enhance the prudent and responsible use of antimicrobial agents in animal health.