Under the auspices of the FAO & OIE Global Framework for the progressive control of Trans-boundary Animal Diseases (GF-TADs), some 70 veterinary and medical professionals and scientists met in Djibouti from April 21 to 23rd, 2015 to reassess the situation of Rift Valley Fever (RVF) on both sides of the Red Sea: “new options for trade, prevention and control”.
The Conference was attended by government representatives of 18 countries including Bahrein, Comoros, Djibouti, Egypt, Ethiopia, Jordan, Kenya, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Somalia, South Sudan, Sudan, Syria, Tanzania and Uganda. The Delegation from Yemen, due to the political situation in the country, could unfortunately not attend the Conference.
Overview of some of participants. Picture © P. Bastiaensen (oie) 2015.
The conference was officially opened by the Djibouti Minister of Agriculture, Livestock, Water and Fisheries, H.E. Mohamed Ahmed Awaleh. Other high profile guests were the Somali Minister of Livestock, Forestry and Range, H.E. Hussein Saïd, the Director of the Agriculture and Environment Division of the Inter-Governmental Authority on Development (IGAD), M. Mohamed Moussa, the FAO Resident Representative, Dr. Emmanuelle Guerne-Bleich, the FAO Regional Representative for the Near East and North Africa, Dr. Markos Tibbo, the OIE Regional Representative for the Middle East, Dr. Ghazi Yehia, the Director of AU-IBAR, Prof. Ahmed El-Sawalhy and the Deputy Director of AU-PANVAC, Dr. Charles Bodjo. The WHO acting Resident Representative, Mrs Houda Langar could not make it to the meeting because of crisis meetings regarding the situation in neighbouring Yemen, but she was ably represented by Dr. Pierre Formenty from the WHO Emerging and Dangerous Pathogens Team in Geneva.
The meeting was organised around 5 formal thematic sessions, interspersed with two working group sessions and a visit to the Djibouti Livestock Export Quarantine Facility, operated by the PRIMA International Company.
The thematic sessions were :
The experts and participants, through formal presentations, case studies and mock negotiations, as well as through the visit of the quarantine station, were given the opportunity to exchange views on the current spread of RVF infection in Western, Southern, Eastern Africa, the Horn of Africa and the Middle-East, the new developments regarding vaccines and diagnostics, but also the delays in the registration, at national levels, of not-so-new vaccines such as the “Clone 13” vaccine, the consequences of the revised Code Chapter on RVF for international trade in live animals, issues of certification and transparency, forecasting and disease preparedness, as well as regional initiatives in support of Rift Valley fever control at regional (IGAD, African Union) and international (GF-TADs).
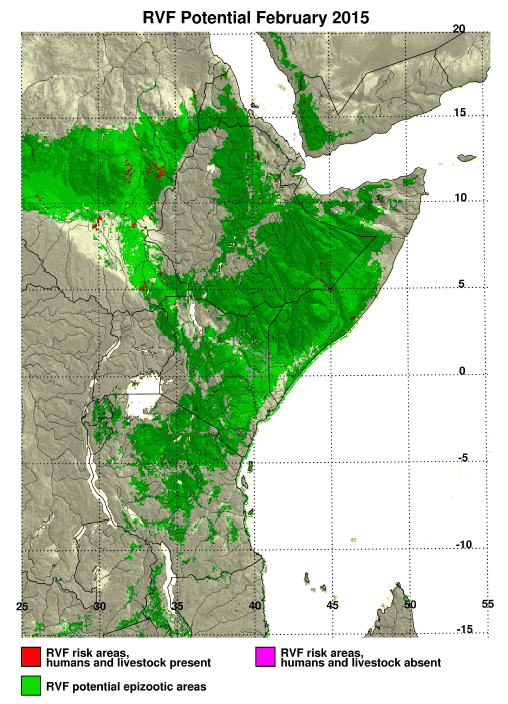
RVF Rift risk map east Africa 2015
Map courtesy of Assaf Anyamba and NASA
Major discussions evolved around new vaccines with the potential to reconcile prophylaxis with the maintenance of trade (DIVA vaccines : differentiating infected from vaccinated animals), the escalating costs and dwindling availability of commercial diagnostic kits, the imminent threat of a new epizootic phase in countries such as South Sudan, Kenya, Tanzania, and others, based on the fact that 2015 is year 8 after the last outbreaks that occurred in the region and that the ENSO prediction model (El-Nino Southern Oscillation) shows a consistent increase in chances of climate abnormalities occurring towards the end of this year (2015).
Participants recommended making use of all available information and tools, including the recently updated Decision support framework for Rift Valley fever and the WHO-OIE Operational Framework on Good Governance of human and animal heath services (download below).
Inputs into the meeting were provided by the African Union (AU-IBAR and AU-PANVAC) and the Inter-Governmental Authority on Development (IGAD), the regional economic community for the Horn of Africa, along with speakers from international organisations such as FAO, OIE, WHO and ILRI, and private and public stakeholders in research and trade, such as CDC-Kenya (Kenya), CVI Lelystad (Netherlands), Deltamune (South Africa), GALVmed (UK), KEMRI (Kenya), OBP (South Africa), ARC-OVI (South Africa), MCI (Morocco) and NASA (US).
The organisers were grateful for the financial support provided by the Governments of Great Britain (UK) and the United States (US) through the OIE World Animal Health and Welfare Fund and the AU-IBAR Standard Methods and Procedures in Animal Health project (SMP-AH) respectively. The considerable support provided by PRIMA International C° was equally acknowledged.
All pictures © P. Bastiaensen (oie) 2015, except where mentioned otherwise.